|
CHEMISTRY TODATY
WELCOME TO CHEMISTRY WORLD
Friday, March 11, 2011
EAT LOTS OF SWEET POTATOES
Tuesday, March 8, 2011
ABOUT CHEMISTRY
Our entire universe is made up of matter which is constantly changing forms and evolving into other forms of energy. Chemistry is defined as the study or science of this ever changing matter. The other sciences which we study commonly like biology, physics and mathematics are all dependent on chemistry and are known as specific studies under the elaborate subject of chemistry. Since there ischemistry seen in biological forms as well as physical states of nature, there are subjects called biochemistry and physical chemistry which help study these changes. There are many chemical changes which occur around us everyday but we are never aware of them. But this is a great way of teaching children how magical the world of chemistry is! With these real examples you can teach them by taking a chemistry in everyday lifequiz, as practical studies are always fun to learn. To make this job easier for you, mentioned below are a few such examples of chemistry in everyday life, take a look!
CHEMISTRY IN EVERY DAY LIFE
Introduction
Study of chemistry is important for the simple reason that, many chemicals find applications in almost all aspects of our daily life.
Chemotherapy
Chemotherapy is the use of chemicals or drugs to selectively destroy infectious micro-organisms without destroying the live tissues or the host. Paul Ehrlich called drugs as magic bullets and the first milestone of his research was the discovery of Salvarsan for curing syphilis, in 1909. In 1935, Gerhard Domagk, administered a dose of a dye called prontosil (inhibits the growth of streptococci bacteria) to cure his daughter's fever. This laid the foundation for modern chemotherapy and got a Nobel Prize for medicine for Domagk in 1939. Ernest Fourneau, a French scientist in 1936 proved that in the human body, prontosil breaks down to give sulphanilamide. Sulphanilamide is the actual active agent that inhibits streptococci. This study led to the discovery of sulpha drugs and from there on growth of chemotherapy has reached amazing heights.
Analgesics
Narcotics
These analgesics are mainly opium and its products. Some examples are morphine, codeine and heroin. They are effective analgesics but cause addiction. Over dosage can cause sleep and unconsciousness.



These analgesics are mainly opium and its products. Some examples are morphine, codeine and heroin. They are effective analgesics but cause addiction. Over dosage can cause sleep and unconsciousness.



Tranquillisers
Tranquillisers reduce anxiety and tension. They are of two types:
a) Sedatives
b) Antidepressants (mood elevators or Pep pills)
a) Sedatives
b) Antidepressants (mood elevators or Pep pills)
Antiseptics and Disinfectants
Sterilization is the process of complete elimination of micro-organisms. The chemicals used for sterilization are classified as:
a) Antiseptics
b) Disinfectants
a) Antiseptics
b) Disinfectants
Anti-fertility Drugs
With global population growing by the day, birth control has become essential. There are drugs that control ovulation and if regularly consumed, function as effective contraceptives. Some examples of birth control pills are orthonovum and Enovid. Orthonovum is a mixture of norethindrone (17a - ethynyl - 19 - nortestosterone) and mestranol (17a - ethynyl - 3 - methoxy - 1,3,5(10), estratriene - 17 b - ol). Envoid is a mixture of norethynodrel (17 a - ethynyl - 17 - b - hydroxy - 5 (10)-estern - 3-one) and mestranol.
Antacids
Tension and mental stress escalate the level of acid in bile juice. This hyperacidity can be combated using bases like calcium carbonate, magnesium hydroxide or aluminium hydroxide in the form of tablets or aqueous suspensions. These react with hydrochloric acid in the stomach and neutralize it partially. Gelusil and Digene are two examples of antacids.
Antihistamines
Histamine is naturally present in almost all body tissues. When the human body meets substances causing allergies, histamine is released. For e.g., when a person is suffering from hay fever, histamine is released. Amines that are used as drugs to control the allergy caused by histamines are calledAntihistamines.
Antibiotics
They are produced by micro-organisms that are toxic to other micro organisms. Alexander Fleming in 1920 found that bacteria donot flourish in nutrient agar surrounded by the fungus Penicillium notatum westling. He found that this fungus produces an antibiotic called penicillin. There are many varieties of pencillin with the empirical formula C9H11O4SN2R.
Dyes - Meaning and Characteristics
Colored substances used for dyeing fabrics are called dyes. A true dye must:
* Have a suitable color
* Be able to attach itself to the material from solution or be capable of being fixed on it
* Be fast to light and washing when fixed. For this it must be resistant to water, acid and alkali
* Have a suitable color
* Be able to attach itself to the material from solution or be capable of being fixed on it
* Be fast to light and washing when fixed. For this it must be resistant to water, acid and alkali
Dyes - Chromophores
Unsaturated groups or groups with multiple bonds that impart color to the organic compound are called chromophores. Examples are the nitro, the nitroso and the azo groups.
Auxochromes as Dyes
Auxochromes (salt forming groups like hydroxyl, amino) do not impart color to the chromogens in the absence of chromophores. However, when the chromogen has a chormophore, the auxochrome deepens the color of the chromogen. It is also used to make the chromogen a dye.
Classification of Dyes Based on Chemical Structure
Oldest synthetic dyes do not have much commercial importance.


Classification of Dyes Based on Application
Direct or Substantive Dyes
These can be directly applied by immersing the cloth in a hot solution of the dye in water. They can be again classified into acid and basic dyes.
Acid dyes are sodium salts of sulphonic acid and nitrophenols. They are used for dyeing animal fibers (wool and silk) but not vegetable fibers (cotton). The dye solution is acidified with sulphuric or acetic acid.
Basic dyes are salts of color bases with hydrochloric acid or zinc chloride. They can directly dye animal fibers. They need a fixing agent called mordant (tannin) to dye vegetable fibers. These are used for dyeing silk and cotton.
These can be directly applied by immersing the cloth in a hot solution of the dye in water. They can be again classified into acid and basic dyes.
Acid dyes are sodium salts of sulphonic acid and nitrophenols. They are used for dyeing animal fibers (wool and silk) but not vegetable fibers (cotton). The dye solution is acidified with sulphuric or acetic acid.
Basic dyes are salts of color bases with hydrochloric acid or zinc chloride. They can directly dye animal fibers. They need a fixing agent called mordant (tannin) to dye vegetable fibers. These are used for dyeing silk and cotton.
Methyl Orange as a Dye
This belongs to the azodyes. It is prepared by coupling diazotized sulphanilic acid with dimethylaniline.


Aniline Yellow (Amino azobenzene) as a Dye
This is another azodye and has little value as a dye. This is because it is sensitive to acids. This is the simplest basic azo dye. This can be obtained by coupling benzene diazomium chloride with aniline.




Malachite Green as a Dye
Belongs to the triphenyl methane dyes. Prepared by condensing 1 molecule of benzaldehyde with 2 molecules of dimethylaniline (1:2 ratio) in presence of con H2SO4. The leuco base is oxidised with lead dioxide and HCl to color base which further reacts with HCl to give the dye.
Natural Dyes (Alizarin and Indigo)
Dyes can also be classified as natural and synthetic dyes. Compounds extracted from plants are called natural dyes. These were used in olden days to color fabrics. Alizarin (red) and indigo (blue) are two examples. Synthetic dyes came into being to provide more varieties of colors.
Chemicals in Cosmetics
Chemicals find great use in cosmetics. Creams like cleansing creams, cold creams, bleaching and vanishing creams are prepared synthetically from chemicals. Perfumes, talcum powders and deodorants are also some other cosmetic substances that are obtained from chemicals. Lipsticks, nail polish and hair dyes also are chemical substances.
Perfumes
Perfumes have pleasant smell due to the esters used in their synthesis.
Carbon Fibres
Carbon fibers are made of long chain of carbon atoms.
They are got from synthetic or regenerated fibers by heating them in the absence of oxygen. These fibers on heating decompose to produce carbon fibers.
They are got from synthetic or regenerated fibers by heating them in the absence of oxygen. These fibers on heating decompose to produce carbon fibers.
Ceramics
Besides being useful, chemicals find use in artifacts as well. Ceramics, paints, varnishes, glass, cement are various other useful substances that contain various chemicals as their components. Construction industry is the major beneficiary of such substances.
Micro Alloys
Micro alloyed steels are intermediate carbon steel alloys with 0.3 to 0.6% carbon content. They also include vanadium, columbium (niobium), titanium and so on. These micro alloys are tougher than higher alloys. Their enhanced strength is due to the precipitation hardening reaction where nitrides or carbonitrides are formed in steel. Therefore, nitrogen level control is a key factor.
Importance of Chemicals in Food
Actually speaking, natural food substances are various forms of chemicals. For e.g., rice is a carbohydrate. Fruits contain carbohydrates and acids like citric acid. Vegetables contain proteins (amino acid blocks) and vitamins. Besides these, chemicals are also used as preservatives for canned or bottled food items to increase their shelf life. Chemicals also find use as edible colors and artificial sweetening agents.
Chemical Preservatives
Chemicals added to food materials to prevent the growth of micro organisms or prevent spoilage and to increase their shelf life are called preservatives.
Artificial Sweetening Agents
For diabetic patients, sugar cannot be used as a sweetening agent. Artificial sweetening agents that are non-nutritive in nature are used as substituents for sugar (specially in soft drinks). Examples are saccharin (500 times sweeter than sucrose) and cyclamates.
Edible Colours and Flavours
Food colors are used in ice creams, dairy products, sweet meat, soft drinks, confectionery, etc. These colors are also used in oral medicines like capsules, tablets, syrups and liquids to improve their appearance. Some of the primary colors are water soluble. They are: quinoline yellow, tartrazine, sunset yellow FCF, erythrosine, poncean 4R, carmoisine, amaranth and brilliant blue.
Soaps and Detergents
Soaps are sodium or potassium salts of higher fatty acids like stearic, palmitic and oleic acids. Fatty acids are organic acids that have more than sixteen carbon atoms in their molecular structure. The sodium soaps are called hard soaps and the potassium soaps are known as soft soaps. Soaps are obtained from oils and fats. For e.g., tristearin is got from beef and mutton tallow, tripalmitin from palm oil and triolein from lard (pig fat), olive oil and cotton seed oil. In India, soap is commonly got from coconut, groundnut, til and mahua oils.
Synthetic Detergents
They possess the desirable properties of ordinary soaps and can be used with hard water and in acidic solutions as well. Synthetic detergents are sodium salts of long chain benzene sulphonic acids or sodium salt of long chain alkyl hydrogen sulphates. Their calcium or magnesium salts are soluble in water.
Rocket Propellants
Propellants are the fuels used in rockets for propulsion. For example, alcohol, liquid hydrogen, liquid ammonia, kerosene, hydrazine and paraffin can be used as propellants.
Insect Repellents
The chemicals like dimethyl phthalate, N, N-diethyl - meta - toulamide (Deet), N - N - diethyl benzamide are used as effective repellents against mosquitoes, flies and other insects. These are widely used in insect repellant body creams.
 Pheromones or Sex Attractants
Pheromones or Sex Attractants
Another way to get rid of insects is to use pheromones or insect sex attractants. These chemicals help induce the mating urge and attract insects of opposite sex. When coated on poisonous baits, they prove fatal for insects. Methyl engenol attracts the oriental fruit fly. Bombykol attracts the silk worm moth.
Summary
Our body is made up of tissues, which are all composed of chemicals. We need an adequate supply of chemicals in the form of food, vitamins, hormones, and enzymes, which are in turn chemicals. For taking care of our health we need medicines. We find that chemicals and chemistry penetrate intoevery aspect of our life. Paper, sugar, starch, vegetable oils, ghee, essential oils, tannery, distillery, soap, cosmetics, rubber, dyes, plastics, petroleum infact there is almost nothing that we use in our dailylife that is not a chemical. Continuing research will keep adding to this list.
Friday, February 18, 2011
prepare nano materials
LETTER FROM RK SIVAKUMAR TO ME :
good afternoon ,this method is very easy and cheaf.we prepare nano materials by using
deionised water.we can prepare ag,zr,co,cr,al,zn nano material by this method.
the closed chamber was then placed inside the a preheated box furnace and the mixture was heated slowly
2 c/min to 200 c and maintained the temp for 612 hours.
mechasim:
Agno3+nabh 4 ? Ag +no2+water
good afternoon ,this method is very easy and cheaf.we prepare nano materials by using
deionised water.we can prepare ag,zr,co,cr,al,zn nano material by this method.
- 3 mg of metal powder was (like Ag ions) added to 30 ml deionized water in a glass veil
- the reaction mixture was sonicated for about 15 min in a glass,transfered in to a stainless steel teflon linked metallic
the closed chamber was then placed inside the a preheated box furnace and the mixture was heated slowly
2 c/min to 200 c and maintained the temp for 612 hours.
- the furnace is allowed to cool after the desired time and the suspension was centrifuged to retrive the product,washed
mechasim:
Agno3+nabh 4 ? Ag +no2+water
Tuesday, December 28, 2010
Tomato
Tomato
The tomato is a plant in the Solanaceae or nightshade family. The taxonomic name is eitherSolanum lycopersicum or Lycopersicon esculentum depending on the reference. Originating in South and Central America, the tomato is now grown world-wide for its brightly coloured (usually red, from the pigment lycopene) edible fruits. The word tomato derives from Náhuatltomatl
| Contents |
Pre-history
The tomato is believed to have been first cultivated in ancient Peru, where several wild species of green tomatoes still grow. Then about three thousand years ago it was brought to Mexico. It is an offshoot of the Mexican lineage L. esculentum cerasiforme which is thought to be the direct ancestor of the modern tomato. The pottery of ancient Peruvian city-states do not appear to mention the tomato, this has led some botanists to conclude that the cultivation of the tomato was done in Mexico. However this is not conclusive as many other fruits in continuous cultivation in Peru are not present in the pottery. Also much horticultural knowledge was lost after the arrival of Europeans, and the Christian Church had a policy of burning all pagan books and quartering their keepers.
Early history
In the 16th and 17th centuries, many Europeans believed tomatoes were poisonous because of the plant's relationship to nightshade and tobacco, although they were grown as garden ornamentals.
The first traces of use of tomato as food date back to South Europe in the first half of the 18th century. Only in the second half of the 19th century cultivation of the tomato as food begins to be widespread, mainly in southern Italy and in France.
Vincenzo Corrado, a cook in the Neapolitan court, describes recipes with tomatoes in the bookIl cuoco galante, first edition 1773, adding more recipes with tomatoes in the 1819 edition.
In 1809, Nicolas Appert, a chef from Paris, published L'art de conserver le substances alimentaires d'origine animale et végétale pour plusieurs années, a book on food conservation where he deals also with preserving tomato.
Thomas Jefferson was a pioneer in growing tomatoes, beginning in 1809. He grew large ribbed "Spanish" tomatoes. Jefferson's daughters left numerous recipes that involved tomatoes, including gumbo soups, cayenne-spiced tomato soup, green tomato pickles, tomato preserves, and tomato omelettes. Tomatoes were purchased in 1806 for Presidential dinners. Randolph'sThe Virginia Housewife has seventeen recipes for tomatoes, including gazpacho, gumbo, and catsup. In an 1824 speech before the Albemarle Agricultural Society, Jefferson's son-in-law, Thomas Mann Randolph discussed the transformation of Virginia farming due to the introduction of new crops. He mentioned how tomatoes were virtually unknown ten years earlier, but by 1824 everyone was eating them because they believed they kept one's blood pure in the heat of summer."[1]
The following story is widely cited, but there are doubts by many historians that it ever happened. Some lingering doubts about the safety of the tomato in the United States were largely put to rest in 1820, when Colonel Robert Gibbon Johnson announced that at noon onSeptember 28, he would eat a basket of tomatoes in front of the Salem, New Jerseycourthouse. Reportedly, a crowd of more than 2,000 persons gathered in front of the courthouse to watch the poor man die after eating the poisonous fruits, and were shocked when he lived.
Modern uses of tomatoes
Tomatoes are now eaten freely in Europe as well as in the rest of the world; in fact, periodically since their exoneration, they have been esteemed as a purported aphrodisiac. Today, their consumption is believed to benefit the heart.
Lycopene, one of nature's most powerful antioxidants, is present in tomatoes and has been found to be beneficial in preventing prostate cancer, among other things.
Botanically a berry, the tomato is generally thought of and used as a vegetable: it's more likely to be part of a sauce or a salad than eaten whole as a snack, let alone as part of a dessert (though, depending on the variety, they can be quite sweet, especially roasted).
Tomatoes are used extensively in Mediterranean and Middle Eastern cuisines, especially Italianones. The tomato has an acidic property that is used to bring out other flavors. This same acidity makes tomatoes especially easy to preserve in home canning as tomato sauce or paste.Tomato juice is often canned and sold as a beverage. Unripe green tomatoes can also be used to make salsa, or they can be batter-dipped and fried.
The town of Buñol, Spain annually celebrates La Tomatina, a festival centered on an enormous tomato fight. Tomatoes are also a popular "non-lethal" throwing weapon in mass protests, and there is a common tradition of throwing rotten tomatoes at bad actors or singers on a stage.
Controversies
Fruit or vegetable?
Botanically speaking a tomato is the ovary, together with its seeds, of a flowering plant. This would mean that technically it would be considered a fruit. However, speaking from a culinary perspective the tomato is typically served as or part of a main course of a meal meaning that it would be considered a vegetable. This argument has lead to actual legal implications in the United States. In 1887, U.S. tariff laws which imposed a duty on vegetables but not on fruits caused the tomato's status to become a matter of legal importance. The U.S. Supreme Courtsettled this controversy in 1893, declaring that the tomato is a vegetable, along withcucumbers, squashes, beans, and peas, using the popular definition which classifies vegetables by use: they are generally served with dinner and not dessert. The case is known as Nix v. Hedden
In concordance with this classification, the tomato is the state vegetable of New Jersey
The pronunciation conundrum
In some English speaking countries, the pronunciation of tomato is in dispute: it can either be pronounced to-MAY-to or to-MAH-to. The difference is inherent in the dialects: British Englishspeakers typically favor to-MAH-to, while American English speakers have a tendency to say to-MAY-to. The word's multiple pronunciations were immortalized in song in Gershwin's 1937 song,Let's Call the Whole Thing Off (You say to-may-to and I say to-mah-to / you say po-tay-to and I say po-tah-to), and have become a symbol for nitpicking pronunciation disputes. In this capacity it has even become an American slang term: saying "to-may-to, to-mah-to" when presented with two choices can mean "what's the big deal, there's no real difference."
Proper storage
Many people believe that tomatoes should be stored refrigerated. This actually destroys the flavor and texture. Ideally tomatoes should be stored between 55–65°F (13–18°C) at 80–95% relative humidity.
Picking and ripening
Tomatoes sold in American grocery stores are often picked unripe, and ripened in storage withethylene. Ethylene is the plant hormone produced by many fruits and acts as the cue to begin the ripening process. These tend to keep longer — but have poorer flavor and a mealier, starchier texture than tomatoes ripened on the plant. They may be recognized by their color, which is more pink or orange than the ripe tomato's deep red.
Recently, stores have begun selling "tomatoes on the vine" which are ripened still connected to a piece of vine. These tend to be much more flavorful (at a price premium) than artificially-ripened tomatoes, but still may not be the equal of local garden produce.
Also relatively recently, slow-ripening varieties of tomato have been developed by crossing a non-ripening variety with ordinary tomato varieties. Varieties were selected whose fruits have a long shelf life and at least reasonable flavor
Bananas Are Radioactive
Did you know bananas are slightly radioactive? Bananas contain high levels of potassium. Radioactive K-40 has an isotopic abundance of 0.01% and a half-life of 1.25 billion years. The average banana contains around 450 mg of potassium and will experience about 14 decays each second. It's not something you need to worry about, since 0.01% of the potassium already in your body is K-40, plus the element is essential for proper nutrition. If you have a banana for your lunch you aren't going to set off a Geiger counter. If you carry a produce truck full of them, then you might have a noticeable radiation signature. The same is true if you are carrying a lot of potatoes or potassium fertilizer.
Friday, December 3, 2010
H2O
Water is, in fact, a chemical. Its chemical formula is H2O (or, less
commonly, HOH), which is what this website is named after. H2O is
also one of the most well-known chemical formulas. When properly
written, the "2" after the H is written in subscript (as you can see in the
banner above), but due to formatting restrictions it will simply be
written as "H2O" on this website.
What does H2O mean? Each water molecule is made of two hydrogen
atoms and one oxygen atom, thus there are two "H" atoms and one
"O". The atoms are joined by covalent bonding, meaning that they
share electrons (as opposed to ionic bonding, in which atoms
completely transfer electrons). On the right is an image of water's
molecular structure.
Water is the most abundant molecule on Earth. Approximately 70% of
the Earth's surface is water. Water is also the only substance on Earth
which naturally occurs in a solid, liquid and gas form.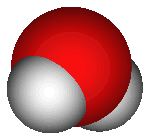
The Celsius temperature scale is based on waters' freezing point (0
degrees) and boiling point (100 degrees). Unlike most substances,
water's solid from is less dense than its liquid form - this is why ice
cubes will float in your drinks. Water is also capable of absorbing a lot
of heat before its temperature increases; thus it is used in things such
as a radiator coolant in cars.
Water has a high surface tension. This is why some bugs like water
striders (of the Gerridae family) can walk on water - because they weigh
less than the surface tension of the water. Due to the shape of a water
molecule, molecules stick and clump together to form this high
tension (the two lighter hydrogen atoms in the image on the right
could attach to the oxygen atom of another water molecule, and so
forth). That's why water comes together in the form of drops - if it wasn't
for gravity water would attach together in a spherical shape.
commonly, HOH), which is what this website is named after. H2O is
also one of the most well-known chemical formulas. When properly
written, the "2" after the H is written in subscript (as you can see in the
banner above), but due to formatting restrictions it will simply be
written as "H2O" on this website.
What does H2O mean? Each water molecule is made of two hydrogen
atoms and one oxygen atom, thus there are two "H" atoms and one
"O". The atoms are joined by covalent bonding, meaning that they
share electrons (as opposed to ionic bonding, in which atoms
completely transfer electrons). On the right is an image of water's
molecular structure.
Water is the most abundant molecule on Earth. Approximately 70% of
the Earth's surface is water. Water is also the only substance on Earth
which naturally occurs in a solid, liquid and gas form.

The Celsius temperature scale is based on waters' freezing point (0
degrees) and boiling point (100 degrees). Unlike most substances,
water's solid from is less dense than its liquid form - this is why ice
cubes will float in your drinks. Water is also capable of absorbing a lot
of heat before its temperature increases; thus it is used in things such
as a radiator coolant in cars.
Water has a high surface tension. This is why some bugs like water
striders (of the Gerridae family) can walk on water - because they weigh
less than the surface tension of the water. Due to the shape of a water
molecule, molecules stick and clump together to form this high
tension (the two lighter hydrogen atoms in the image on the right
could attach to the oxygen atom of another water molecule, and so
forth). That's why water comes together in the form of drops - if it wasn't
for gravity water would attach together in a spherical shape.
Tuesday, November 30, 2010
Citric acid
Citric acid
General | |
|---|---|
| Name | Citric acid |
| Chemical formula | C6H8O7 |
| Formula weight | 192.13 amu |
| Synonyms | 2-hydroxy-1,2,3-propanetricarboxylic acid |
| CAS number | 77-92-9 |
Phase behavior | |
| Melting point | 426 K (153 °C) |
| Thermal decomposition temperature | 448 K (175°C) |
Acid-base properties | |
| pKa1 | 3.15 |
| pKa2 | 4.77 |
| pKa3 | 5.19 |
Solid properties | |
| ΔfH0 | -1543.8 kJ/mol |
| S0 | 252.1 J/mol·K |
| Cp | 226.5 J/mol·K |
| Density | 1.665 ×103 kg/m3 |
Safety | |
| Acute effects | Skin and eye irritant. |
| Chronic effects | None. |
More info | |
| Properties | NIST WebBook |
| MSDS | Hazardous Chemical Database |
Citric acid is a weak organic acid found in citrus fruits. It is a good, naturalpreservative and is also used to add an acidic (sour) taste to foods and soft drinks. In biochemistry, it is important as an intermediate in the citric acid cycle and therefore occurs in the metabolism of almost all living things. It also serves as an environmentally friendly cleaning agent and acts as an antioxidant.
Citric acid exists in a variety of fruits and vegetables, but it is most concentrated inlemons and limes, where it can comprise as much as 8% of the dry weight of the fruit.
Citric acid's chemical formula is C6H8O7 and its structure is shown at right. This structure is reflected in its IUPAC name 2-Hydroxy-1,2,3-propanetricarboxylic acid.
| Contents [hide] |
Properties
The physical properties of citric acid are summarized in the table at right. The acidity of citric acid results from the three carboxy groups COOH which can lose aproton in solution. If this happens, the resulting ion is the citrate ion. Citrates make excellent buffers for controlling the pH of acidic solutions.
Citrate ions form salts called citrates with many metal ions. An important one iscalcium citrate or "sour salt", which is commonly used in the preservation and flavoring of food. Additionally, citrates can chelate metal ions, which gives them use as preservatives and water softeners.
At room temperature, citric acid is a white crystalline powder. It can exist either in an anhydrous (water-free) form, or as a monohydrate that contains one water molecule for every molecule of citric acid. The anhydrous form crystallizes from hot water, while the monohydrate forms when citric acid is crystallized from cold water. The monohydrate can be converted to the anhydrous form by heating it above 74°C.
Chemically, citric acid shares the properties of other carboxylic acids. When heated above 175°C, it decomposes through the loss of carbon dioxide and water.
History
The discovery of citric acid has been credited to the 8th century Islamic alchemistJabir Ibn Hayyan (Geber). Medieval scholars in Europe were aware of the acidic nature of lemon and lime juices; such knowledge is recorded in the 13th centuryencyclopedia Speculum Majus (The Great Mirror), compiled by Vincent of Beauvais. Citric acid was first isolated in 1784 by the Swedish chemist Carl Wilhelm Scheele, who crystallized it from lemon juice. Industrial-scale citric acid production began in 1860, based on the Italian citrus fruit industry.
In 1893, C. Wehmer discovered that Penicillium mold could produce citric acid fromsugar. However, microbial production of citric acid did not become industrially important until World War I disrupted Italian citrus exports. In 1917, the Americanfood chemist James Currie discovered that certain strains of the mold Aspergillus niger could be efficient citric acid producers, and Pfizer began industrial-level production using this technique two years later.
Production
In this production technique, which is still the major industrial route to citric acid used today, cultures of Aspergillus niger are fed on sucrose to produce citric acid. After the mold is filtered out of the resulting solution, citric acid is isolated by precipitating it with lime (calcium hydroxide) to yield calcium citrate salt, from which citric acid is regenerated by treatment with sulfuric acid.
Alternatively, citric acid is sometimes isolated from the fermentation broth byextraction with a hydrocarbon solution of the organic base trilaurylamine , followed by re-extraction from the organic solution by water.
Uses
Most citric acid is used as a flavoring and preservative in food and beverages, especially soft drinks; it is denoted by E Number E330. Citrate salts of various metals are used to deliver those minerals in a biologically available form in many dietary supplements. The buffering properties of citrates are used to control pH in household cleaners and pharmaceuticals.
Citric acid's ability to chelate metals gives it use in soaps and laundry detergents. By chelating the metals in hard water, it lets these cleaners produce foam and work better without need for water softening. Similarly, citric acid is used to regenerate the ion exchangematerials used in water softeners by stripping off the accumulated metal ions as citrate complexes.
It is used in the biotechnology and pharmaceutical industry to passivate high purity process piping in lieu of using nitric acid, since nitric is a hazardous disposal issue once it is used for this purpose, while citric is not.
In the United Kingdom, pharmacies control the sale of Citric acid. Citric acid is a popular buffer used to increase the solubility of streetheroin in Scotland. Single-use citric acid sachets have been used as an inducement to get heroin users to exchange their dirty needles for clean needles in an attempt to decrease the spread of AIDS and hepatitis. See the .pdf article here. Other acidifiers used for brown heroin are ascorbic acid, acetic acid, and lactic acid: in their absence, the drug abuser will often substitute lemon juice or vinegar.
Safety
Citric acid is recognized as safe for use in food by all major national and international food regulatory agencies. It is naturally present in almost all forms of life, and excess citric acid is readily metabolized and eliminated from the body.
Subscribe to:
Posts (Atom)




