commonly, HOH), which is what this website is named after. H2O is
also one of the most well-known chemical formulas. When properly
written, the "2" after the H is written in subscript (as you can see in the
banner above), but due to formatting restrictions it will simply be
written as "H2O" on this website.
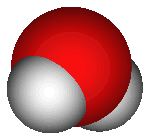
What does H2O mean? Each water molecule is made of two hydrogen
atoms and one oxygen atom, thus there are two "H" atoms and one
"O". The atoms are joined by covalent bonding, meaning that they
share electrons (as opposed to ionic bonding, in which atoms
completely transfer electrons). On the right is an image of water's
molecular structure.
Water is the most abundant molecule on Earth. Approximately 70% of
the Earth's surface is water. Water is also the only substance on Earth
which naturally occurs in a solid, liquid and gas form.
The Celsius temperature scale is based on waters' freezing point (0
degrees) and boiling point (100 degrees). Unlike most substances,
water's solid from is less dense than its liquid form - this is why ice
cubes will float in your drinks. Water is also capable of absorbing a lot
of heat before its temperature increases; thus it is used in things such
as a radiator coolant in cars.
Water has a high surface tension. This is why some bugs like water
striders (of the Gerridae family) can walk on water - because they weigh
less than the surface tension of the water. Due to the shape of a water
molecule, molecules stick and clump together to form this high
tension (the two lighter hydrogen atoms in the image on the right
could attach to the oxygen atom of another water molecule, and so
forth). That's why water comes together in the form of drops - if it wasn't
for gravity water would attach together in a spherical shape.


No comments:
Post a Comment