The chemistry of life

In this lens I'd like to talk you about the Chemistry of daily life. Chemistry is an exciting experimental science which lets us to understand our world and makes our life easier. As you will read in the following articles, chemistry is in our everyday life: in our body, at home, in the nature... in every second of our lives!
If any of you have a question about the chemistry of your everyday life, please feel free tocontact me, and I will publish here the answers to those that may be interesting for most of the people. I invite you also to leave a comment in my guestbook below.
Thank you very much to the Squidoo staff for selecting this lens on April 28th as the 'lens of the day', I feel honoured! I invite you also to visit my other lens: Spain in depth, and my website. Thank you!
Greetings from Spain,
Silvia
How soap cleans?

There are substances which can be dissolved in water (salt for example), and others that can't (for example oil). Water and oil don't mix together, so if we try to clean an oily stain from a cloth or from the skin, water is not enough. We need soap.
Soap is formed by molecules with a "head" which likes water (hydrophilic) and a long chain which hates it (hydrophobic).
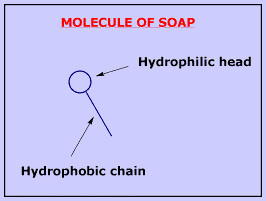
Because of this dualism, soap molecules act like a diplomat, improving the relationship between water and oil. How? When soap is added to the water, the hydrophilic heads of its molecules stay into the water (they like it!), while the long hydrophobic chains join the oil particles and remain inwards (escaping from the water). In that way, they form circular groups named micellas, with the oily material absorbed inside and trapped.
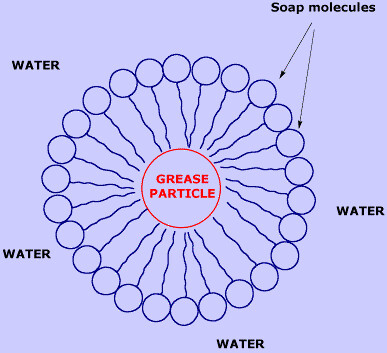
An emulsion of oil in water is then formed, this means that the oil particles become suspended and dispersed into the water. Thus, those oil particles are liberated from the cloth or the skin, and the emulsion is taken away with the rinsing.
In summary, soap cleans by acting as an emulsifier. It allows oil and water to mix so that oily grime can be removed during rinsing.There are more things involved in this process, such as for instance changes in the superficial tension of water, but this is the general idea.
Soap is formed by molecules with a "head" which likes water (hydrophilic) and a long chain which hates it (hydrophobic).

Because of this dualism, soap molecules act like a diplomat, improving the relationship between water and oil. How? When soap is added to the water, the hydrophilic heads of its molecules stay into the water (they like it!), while the long hydrophobic chains join the oil particles and remain inwards (escaping from the water). In that way, they form circular groups named micellas, with the oily material absorbed inside and trapped.

An emulsion of oil in water is then formed, this means that the oil particles become suspended and dispersed into the water. Thus, those oil particles are liberated from the cloth or the skin, and the emulsion is taken away with the rinsing.
In summary, soap cleans by acting as an emulsifier. It allows oil and water to mix so that oily grime can be removed during rinsing.There are more things involved in this process, such as for instance changes in the superficial tension of water, but this is the general idea.
Vegetables and colours - Part 1

White light from the sun contains all the wavelengths, but when it impacts on an object some of its wavelenghts are absorbed and some reflected. An object is coloured because of the light that it reflects. For example red objects reflect 'red' light, which is light with a long wavelength. Many vegetables and fruits are strongly coloured because they contain an especial kind of chemical compounds namedcarotenoids. These compounds have an area called choromophore, which absorbs and gives off particular wavelengths of light, generating the colour that we then perceive.
The chromophore is formed by a sequence of linear carbon-carbon double bonds (represented as C=C), much stronger than simple bonds (represented as C-C), so the atoms remain closer to each other. In general, it's necessary at least seven linear conjugated double bonds for a carotenoid to produce a colour. Besides, the bigger the number of bonds conjugated, the bigger the wavelength of the light absorbed and also the more red the vegetable, as you can see in this picture of the light spectrum:

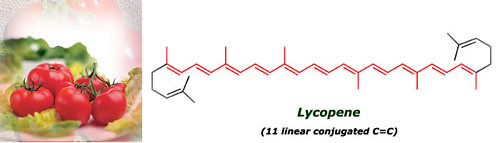
The tomato is red because of the carotenoid lycopene, which contains 11 conjugated carbon-carbon double bonds. You can count these bonds in the picture below, they are selected in red (the atom carbons are omitted, only the bonds are shown). This compound is generated by the plant to protect itself from the air oxidation. So it's a good antioxidant useful for us too, protecting our cells against the action of free radicals (potent oxidants), which are one of the main responsibles of cardiovascular diseases, cancer and aging.
The chromophore is formed by a sequence of linear carbon-carbon double bonds (represented as C=C), much stronger than simple bonds (represented as C-C), so the atoms remain closer to each other. In general, it's necessary at least seven linear conjugated double bonds for a carotenoid to produce a colour. Besides, the bigger the number of bonds conjugated, the bigger the wavelength of the light absorbed and also the more red the vegetable, as you can see in this picture of the light spectrum:

The tomato is red because of the carotenoid lycopene, which contains 11 conjugated carbon-carbon double bonds. You can count these bonds in the picture below, they are selected in red (the atom carbons are omitted, only the bonds are shown). This compound is generated by the plant to protect itself from the air oxidation. So it's a good antioxidant useful for us too, protecting our cells against the action of free radicals (potent oxidants), which are one of the main responsibles of cardiovascular diseases, cancer and aging.

(See the continuation in Part 2 below)
Vegetables and colour - Part 2
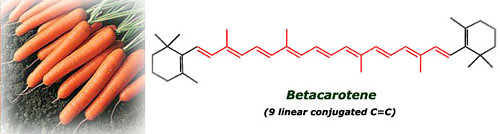
The pigment present in carrots is the betacarotene, with 9 linear conjugated double bonds, less than in lycopene so they are no red but orange (smaller wavelength than red, check it in the spectrum picture). This compound is also a potent antioxidant and besides it's transformed in our body into vitamin A, very important for the maintenance of healthy skin, good vision and a robust inmune system.
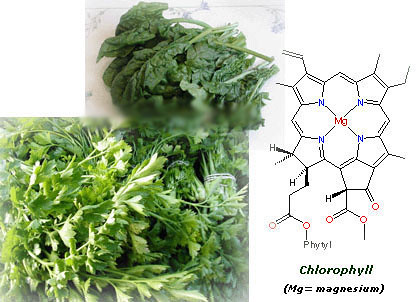
Spinachs, parsley and plants in general are green because they contain chlorophyll, a pigment which enables the plant to carry on photosynthesis, transforming solar energy andcarbon dioxide into chemical energy in the form of carbohydrates and oxygen. This is a process essential for life.
As you can see in the pic below, the structure of chlorophyll is very complicated, so let's simpy say that it contains a big ring with a magnesium atom in the center. Curiously, the structure of hemoglobine (the carrier of oxygen in our blood) is pretty similar to chlorophyll, though it has an atom of iron instead of magnesium in its center.
The chlorophyll masks the other colours in vegetables and as its amount decreases the rest of colours become evident. This explains for example why tomatoes are initially green and then become red when they ripen.
This is an example of how Chemistry is everywhere, sometimes more evident, and sometimes much less :-).

Spinachs, parsley and plants in general are green because they contain chlorophyll, a pigment which enables the plant to carry on photosynthesis, transforming solar energy andcarbon dioxide into chemical energy in the form of carbohydrates and oxygen. This is a process essential for life.
As you can see in the pic below, the structure of chlorophyll is very complicated, so let's simpy say that it contains a big ring with a magnesium atom in the center. Curiously, the structure of hemoglobine (the carrier of oxygen in our blood) is pretty similar to chlorophyll, though it has an atom of iron instead of magnesium in its center.
The chlorophyll masks the other colours in vegetables and as its amount decreases the rest of colours become evident. This explains for example why tomatoes are initially green and then become red when they ripen.

This is an example of how Chemistry is everywhere, sometimes more evident, and sometimes much less :-).

No comments:
Post a Comment